
A young woman working at the Museum of Natural History near CalSci is murdered, leaving behind only a few cryptic equations in a notebook. After Charlie examines the notebook, he realizes that the equations date a new find to 10'000 years old -- a human skull that would be the oldest remains ever found in the area, predating the existing Native American tribe in place by thousands of years.
How do equations combine with the properties Carbon 14 to allow scentists to do Carbon dating?
Carbon 14, also written 14C is a radioactive isotope of carbon that is produced in the upper atmosphere by radiation from the sun: neutrons hit nitrogen-14 atoms and transmute them to carbon.
Trees, flowers and other land plants get the carbon they need to activate the photosynthesis process from carbon dioxide in the air. Some fraction of their carbon is thus 14C, and hence the same is true of all the creatures that get their carbon by eating such plants, or by eating creatures that eat seach plants, and so on. Living organisms remain in a state of equilirium with their environment and hence hold a somewhat predictable quantity of radioactive carbon.

Suppose now that such an organism dies and its body is preserved. The radioactive carbon in its body will undergo radioactive decay and gradually turn back into Nitrogen. Luckily for us, this process is regular enough that we can model its evolution with mathematical equations.
When trying to calculate the age of the sample containing 360 radioactive atoms, you should have noticed that you could only give an approximate age. To try and understand why, we will first simplify the problems a little a bit by choosing different units of atoms and time
Let us thus assume that the number N of atoms is 1 unit at t = 0, and that after 1 unit of time, only one half units remain in the sample. Using carbon dating vocabulary, this says that the half life is now 1 unit of time. We'll try and investigate the value of N(t = 1/2) :

We know that as time passes from t = 0 to t = 1/2, the number N of radioactive atoms gets multiplied by some factor mu1, and that as time passes from t = 1/2 to t = 1, that number gets multiplied by some other factor mu2. But we also know that after one period of time, N is divided by 2.
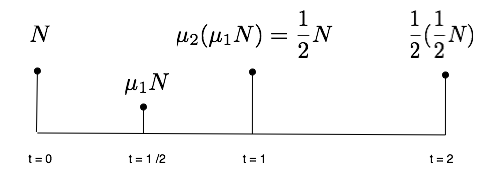
So as long as mu1 mu2 = 1/2, these two numbers can be anything and are not directly specified by the model, at first sight.
This is where we make a choice as mathematicians modeling this problem.
We know that the factor that takes N from t = 0 to t = 1 is the same as that which takes N from t = 1 to t = 2. If we want our model to make sense, it should respect this symmetry and we should have mu1 = mu2 = mu, and thus the following equations:
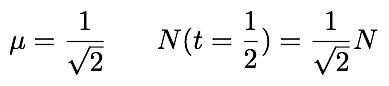
We now apply this knowledge to our original example:
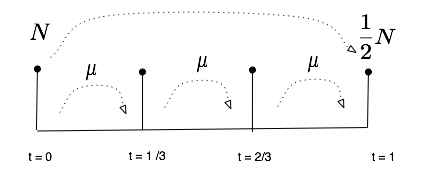
In this section, we investigate what is called a functional equation, that is to say an equation where the unknown is a function and not just a number. Assume here again that the half life is one unit of time, and call N(t) the number of units of atoms in the sample at time t. We will write an equation for N as a function and try to investigate some of its solutions.

The last question makes it obvious now why the radioactive decay of Carbon is called exponential. The number of atoms that decay at a given time is directly proportional to the number of atoms present in the sample.
Another very close example and good application can be found in the theory of compound interest, where the exponiential decay becomes an exponential growth.
The idea here is to model a process where the amount of money available grows by -- say -- 5 % every year. There are many ways of modeling such a process, depending on the properties wanted for the model. To read more about this, check this article